ISO 13485:2003 specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide medical devices and related services that consistently meet customer requirements and regulatory requirements applicable to medical devices and related services.
The primary objective of ISO 13485:2003 is to facilitate harmonized medical device regulatory requirements for quality management systems. As a result, it includes some particular requirements for medical devices and excludes some of the requirements of ISO 9001 that are not appropriate as regulatory requirements. Because of these exclusions, organizations whose quality management systems conform to this International Standard cannot claim conformity to ISO 9001 unless their quality management systems conform to all the requirements of ISO 9001.
All requirements of ISO 13485:2003 are specific to organizations providing medical devices, regardless of the type or size of the organization.
If regulatory requirements permit exclusions of design and development controls, this can be used as a justification for their exclusion from the quality management system. These regulations can provide alternative arrangements that are to be addressed in the quality management system. It is the responsibility of the organization to ensure that claims of conformity with ISO 13485:2003 reflect exclusion of design and development controls.
If any requirement(s) in Clause 7 of ISO 13485:2003 is(are) not applicable due to the nature of the medical device(s) for which the quality management system is applied, the organization does not need to include such a requirement(s) in its quality management system.
The processes required by ISO 13485:2003, which are applicable to the medical device(s), but which are not performed by the organization, are the responsibility of the organization and are accounted for in the organization's quality management system.
Revision information
Revises: ISO 13485:1996
Corrigenda, Amendments and other parts
Source
Medical devices
Cooperating for the safety, quality and performance of medical devices
Introduction to the Global Harmonisation Task Force (GHTF) for Medical Devices, an informal platform for regulatory authorities and representatives of industry from Europe, the United States of America, which goal is to provide a forum for national regulatory authorities and industry representatives in the field of medical devices to promote international convergence in regulatory requirements and practices.
Health care and medical devices
ISO 13485 certification helps Beckman Coulter meet regulatory deadline for medical devices. Beckman Coulter, Inc.,implemented ISO 13485 to meet a new medical device regulation requiring registration to the standard.
Quality management systems for the medical device industry
ISO has just published a standard to facilitate implementation of quality management systems based on ISO 9001:2000 by the medical device industry.
Source
ISO/TR 14969:2004
Medical devices -- Quality management systems -- Guidance on the application of ISO 13485: 2003
Media and price
| Language | Format | Add to basket |
|---|---|---|
| English | PDF (2 444 kB) | CHF 180,00 |
| English | Paper | CHF 180,00 |
| Russian | PDF (911 kB) | CHF 180,00 |
| Russian | Paper | CHF 180,00 |
General information
Number of Pages: 74
| Edition: 1 (Monolingual) | ICS: 03.120.10; 11.040.01 |
| Status: Published | Stage: 90.93 (2007-11-01) |
| TC/SC: TC 210 |
Abstract
ISO/TR 14969:2004 provides guidance for the application of the requirements for quality management systems contained in ISO 13485. It does not add to, or otherwise change, the requirements of ISO 13485. It does not include requirements to be used as the basis of regulatory inspection or certification assessment activities.
This guidance can be used to better understand the requirements of ISO 13485 and to illustrate some of the variety of methods and approaches available for meeting the requirements of ISO 13485.
Revision information
Revises: ISO 14969:1999
These standards could also interest you
- ISO 15225:2010
Medical devices -- Quality management -- Medical device nomenclature data structure - ISO 15223-2:2010
Medical devices -- Symbols to be used with medical device labels, labelling, and information to be supplied -- Part 2: Symbol development, selection and validation - IEC/TR 80002-1:2009
Medical device software -- Part 1: Guidance on the application of ISO 14971 to medical device software
Source
ISO 14971:2007
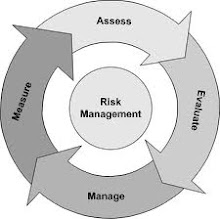
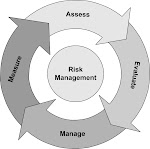
Medical devices -- Application of risk management to medical devices
Media and price
| Language | Format | Add to basket |
|---|---|---|
| English | PDF (1 331 kB) | CHF 192,00 |
| English | Paper | CHF 192,00 |
| French | PDF (1 620 kB) | CHF 192,00 |
| French | Paper | CHF 192,00 |
General information
Number of Pages: 82
| Edition: 2 (Monolingual) | ICS: 11.040.01 |
| Status: Published | Stage: 90.20 (2010-01-15) |
| TC/SC: TC 210 |
Abstract
ISO 14971:2007 specifies a process for a manufacturer to identify the hazards associated with medical devices, including in vitro diagnostic (IVD) medical devices, to estimate and evaluate the associated risks, to control these risks, and to monitor the effectiveness of the controls.
The requirements of ISO 14971:2007 are applicable to all stages of the life-cycle of a medical device.
source















.jpg)





.jpg)

.jpg)









.jpg)

.jpg)














.jpg)


.jpg)


























































































































































































































.jpg)

.jpg)











.jpg)




















































.jpg)
















.jpg)












































































































































.jpg)

























No comments:
Post a Comment