The ISO 9001:2000 Standard has been revised after eight years. And the buzz is rapidly reaching a crescendo. We continue to receive numerous emails and phone calls regarding the impact of the new release. First, let me dispel any anxiety about the November 15, 2008 official “2008″ release. There are no new requirements. The changes are largely interpretive, and focus on terminology clarifications. The changes to the ISO 9001 amendment, however, will have considerable benefit.
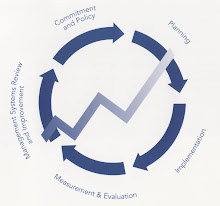
As a back drop, keep in mind that ISO 9001 is not just an International Standard for a Quality Management System. It is the world’s most recognized business management standard. And it makes a strong business case. It focuses attention on leadership,business planning, organizational business processes, your customers (internal and external), measurement and reporting, and continual improvement. That translates to improved business results and a sustainable competitive advantage.
Transitioning to ISO 9001:2008
The International Organization of for Standardization and the International Accreditation Forum have agreed to a smooth transition. Both standards will remain current for two years – until November 15, 2010. Registration of conformity to ISO 9001:2008 will be issued from November 15 on, and after a routine registrar surveillance audit, or a recertification audit against the new version. Also, as of November 15, 2009, all new registrations and recertifications must be to ISO 9001:2008.
Two years after publication of ISO 9001:2008 (November 15, 2010), any certifications that are still to ISO 9001:2000 will no longer be valid. This means that currently certified organizations in “surveillance mode” in 2008-2009 have until November 15, 2010 to be successfully certified to ISO 9001:2008. We recommend a proactive approach. If you can win in your marketplace every day – why put it off?
Overview of the Changes
The points of clarification focus on outsourcing, documentation, management representative, employee competence, design verification and validation, process monitoring, control of nonconforming product and corrective and preventive action. But bear in mind, no “shalls” (requirements) were added or removed.
Examples of select changes include: In an attempt to clarify the term “outsourcing,” notes were added that require the organization to identify processes (and the control of these) to be completed by an external party. With respect to documentation, the changes focus on improving the compatibility between ISO 9001 and ISO 14001.
The only significant addition to the management representative sub clause was to require that the MR be a member of the organization’s management team. This means a contracted person could serve in this role as long as he or she is also considered part of management. The management representative does not have to be full-time.
With regard to competence of employees, a note was added to make training more pervasive throughout the organization. Training applies to all employees directly or indirectly responsible for delivering a service or producing a product (everyone that is part of the quality chain). A complete listing of changes can be found in Annex B (Table B.1) of the Standard. Also, note that there is better alignment with ISO 14001:2004.
Potential Danger and Hidden Opportunities
The Chinese character for crisis consists of two parts – potential danger and hidden opportunities. Clearly, the amended release does not suggest a crisis, and the hidden opportunities far outnumber any potential danger. So, if you are anxious – don’t be. That said, perhaps the greatest potential danger is to dismiss this release, and to rationalize that there is no work to be done.
Even though there are minor changes, hidden opportunities abound. This is the ideal time to be proactive, and to re-energize your quality (business) management system. Ask yourself: Is your QMS integrated with your organization’s vision, mission and values? Are your quality objectives aligned with your organization’s business objectives? Have you considered the needs of your customers in developing your objectives? Are you using a balanced scorecard to evaluate performance (key performance indicators)?
You could drill down further and ask: How are you measuring “the voice of the customer?” Are your document and record control processes effective? Are your preventive and corrective action processes effective? Do you have an effective root cause analysis methodology? Do you have an effective organization-wide training plan? How do you evaluate the competence of your employees? Are you measuring the cost of quality, and driving waste and inefficiency out of your business?
The Path Forward
This pragmatic path forward can be used as a guide to craft a game plan during the transition. It stresses the basic steps that your organization should consider, regardless of the maturity of your QMS.
Obtain a copy of the ISO 9001:2008 Standard. Copies can be purchased from your national standards body ANSI in the United States at (www.ansi.org), the International Organization for Standardization (www.iso.org), or from the American Society for Quality.
Review Annex B, and familiarize yourself with the changes to the Standard. This Annex (Table B.1) identifies the text changes between the 2000 and 2008 versions. It’s a must read.
Discuss the transition requirements with your registrar. Remember you have two years to make the transition. We recommend transitioning sooner than later. This is no time to procrastinate.
Determine whether the changes impact your organization. If nothing else, it will give you and your management team a better understanding of the nuances and underlying reasons for the change.
Use this opportunity to reenergize your QMS. Remember, “ISO” is a fundamental businessmodel, and it is the foundation from which to build a competitive, customer-centric enterprise.
Schedule a meeting with your ISO Business Partner (consulting firm) to assist you with taking your QMS to the next level. Along the way, you should also explore ways to leverage your certificate to accelerate growth of sales.
Source












.jpg)





.jpg)

.jpg)








.jpg)

.jpg)













.jpg)


.jpg)










































































































































































































.jpg)

.jpg)











.jpg)






















































.jpg)















.jpg)



























































































































.jpg)